Navigating narrow paravisceral true lumen in post-dissection thoraco-AAA EVAR
Endovascular repair of post-dissection thoraco-abdominal aortic aneurysms is often challenged by significant compression of the true lumen at the paravisceral segment, causing main graft difficult deployment, cumbersome target vessel cannulation, and limited long-term bridging stent stability.
Different types of aortic stent grafts and bridging stents can be selected to overcome these issues; nevertheless, techniques to favour adequate true lumen expansion during repair may improve short- and long-term outcomes.
A 56-year-old male patient was referred to our center for asymptomatic, extent V-type post-dissection thoraco-abdominal aortic aneurysm. His medical history included hypertension, severe obesity (140 kg), paroxysmal atrial fibrillation, CAD (EF 36 %) and COPD.
Previous medical history
In 2018, the patient was referred to another institution for a complicated acute type B aortic dissection with right renal artery and left lower limb malperfusion. He was emergently treated with TEVAR (Gore C-TAG 36-100 deployed in zone 3) to exclude the sole proximal entry tear, with no additional procedures on the right renal artery or left lower limb.
On the first post-operative day, the patient underwent reintervention for persistent left lower limb ischemia, with a femoro-femoral right-left bypass performed. The post-operative CT angiogram at discharge showed regular exclusion of the proximal entry tear in Zone 3, with residual thoraco-abdominal dissection extended to the aortic bifurcation, with regular patency of celiac trunk (CT), superior mesenteric artery (SMA) and left renal artery (LRA), but complete occlusion of the right renal artery (RRA), with preserved overall renal function and patency of the femoro-femoral bypass.
Current clinical presentation
After a 4-year follow-up, the patient is referred to our institution for post-dissection thoraco-abdominal aneurysmal evolution, with a maximum transverse aortic aneurysm diameter of 6.7 cm at the CT level, associated with true lumen (TL) compression of the paravisceral segment, and a mild infrarenal aortic dilation of 3.3 cm. CT and SMA originated from the TL. RRA was chronically occluded with hypotrophic right kidney, LRA was patent and originated from the false lumen (FL) (Videos 1 and 2, and Figure 1).
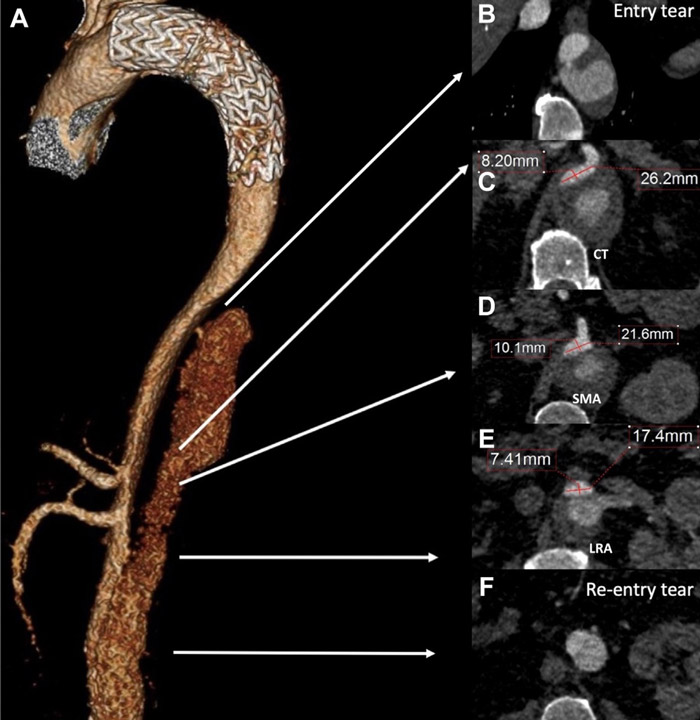
Risk assessment
SVS/AAVS medical comorbidity grading system
- Cardiac status: recurrent congestive heart failure → 3
- Pulmonary status: FEV 1 less than 35 % of predicted value → 3
- Renal status: normal serum creatinine level (single kidney) → 1
- Hypertension: requires more than two drugs to control → 3
- Age: 56 years old → 1
Based on pre-operative assessment, the patient was deemed to be at moderate/severe risk, with an SVS/AAVS score of 2.3.
(0 = no risk, 1 = mild risk, 2 = moderate risk, 3 = severe risk)
Available treatment options
We examined several options, including:
Open surgery:
- thoraco-phrenic laparotomy with surgical septostomy followed by aorto-visceral reconstruction
Endovascular surgery:
- Single-stage total TAAA exclusion
- Dual/ Multiple-stage TAAA exclusion
- TEVAR extension + FEVAR
- TEVAR extension + BEVAR (outer branches)
- TEVAR extension + BEVAR (inner branches)
- TEVAR extension + F/BEVAR
- TEVAR extension + FEVAR or BEVAR or F/BEVAR + additional endo-procedures for narrow paravisceral true lumen management
Final strategy decision
The patient was scheduled for a two-stage endovascular exclusion procedure.
In the first stage, the existing TEVAR was to be extended distally, followed by IVUS-guided electrocautery endo-septostomy of the intimal flap at the paravisceral level.
Subsequently, a standard off-the-shelf preloaded inner branch device was to be deployed. Distally, the procedure was to be completed using a standard EVAR with right distal iliac sealing, employing an appropriately sized iliac limb component.
The distal exclusion of the left limb was intentionally not planned for the first stage to temporarily maintain sac reperfusion and facilitate adequate spinal cord adaptation (Videos 4 and 5).
The second stage, involving the contralateral iliac limb implantation, was planned for 5 weeks later.
Step-by-step summary of the planned procedure
- Bilateral percutaneous common femoral and percutaneous left axillary accesses were obtained.
- Rosen guidewire advanced and parked in LRA from percutaneous left axillary access.
- Distal extention of the previous TEVAR, 5 cm above the CT, just above the entry tear.
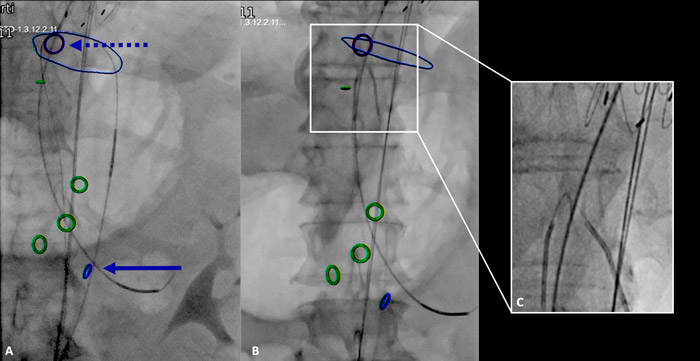
- IVUS-Fusion-guided through-and-through system at the proximal natural entry tear in the distal descending thoracic aorta within the aortic true and false lumen.
- Electrified wire endoseptostomy of the membrane from the proximal to the distal re-entry tear (infrarenal segment).
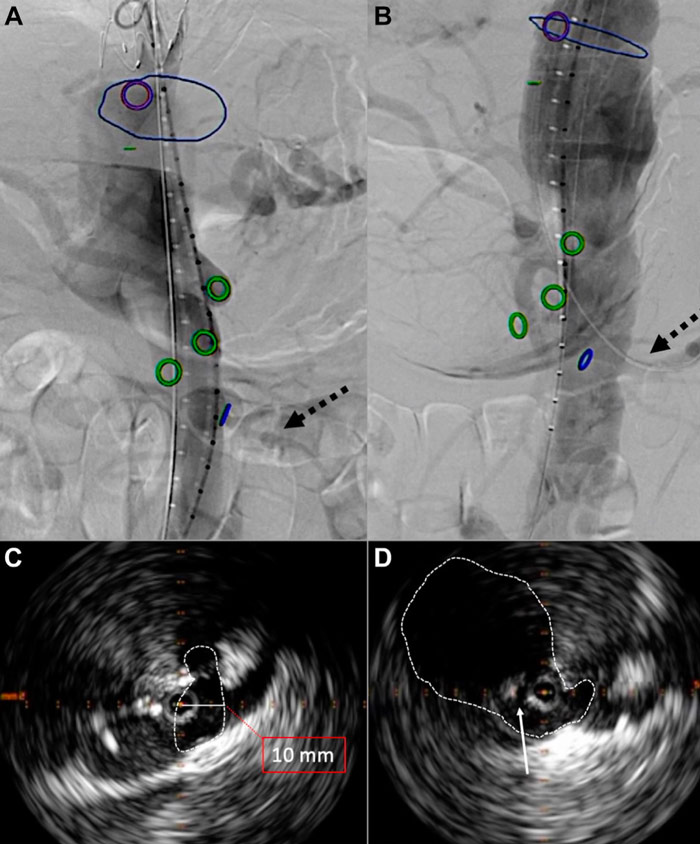
- Double check via IVUS of the origin of major vessels to assess their patency; aortography to evaluate distal visceral and renal vessel perfusion.
- RRA origin embolization.
- E-nside inner branch device deployment from right access.
- Distal EVAR deployment. Only the right distal iliac sealing was completed with iliac limb extension.
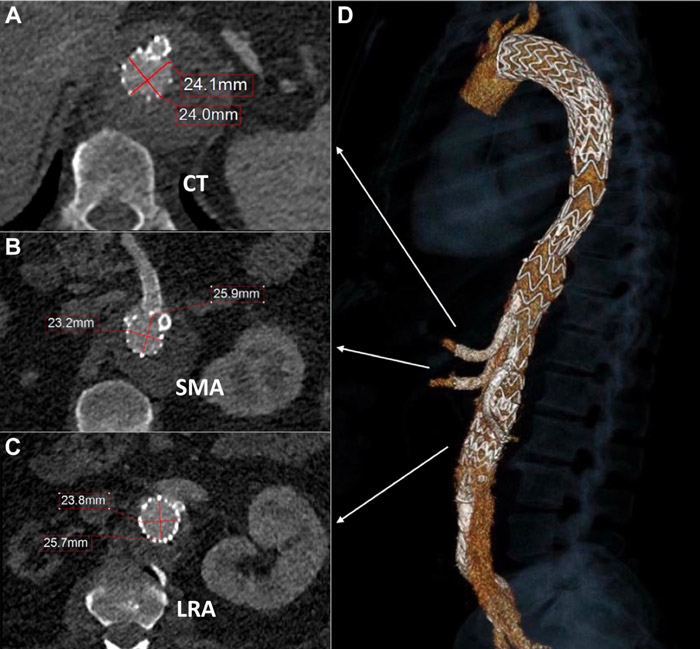
- The CT, SMA, and true lumen of the LRA were sequentially cannulated and stented from right femoral access.
Brief description of each technical step (video 3)
- Under general anesthesia, bilateral percutaneous common femoral and percutaneous left axillary accesses were obtained. Femoral accesses were pre-closed via 2 Proglide devices per side.
- To secure access to the true lumen of the left renal artery throughout the procedure, a Rosen guidewire was advanced and parked in the left renal artery from the percutaneous left axillary access; this was not required for the CT and superior mesenteric artery because they originated from the true lumen (Figure 2).
- The previous TEVAR was extended by deploying an additional TEVAR (28-N4-40-154-36s Bolton Relay Pro).
- Using a combination of IVUS and Fusion TC technique, from bilateral femoral accesses, a through-and-through wire within the aortic true and false was established at the level of the pre-existing proximal re-entry tear. The cheese-wire technique was then performed with an endoseptostomy of the membrane, from the proximal to the distal re-entry tear. The septostomy was performed using a 0.014 Astato XS wire that was angled at the cutting edge and protected on both sides by a 5F ber catheter, one from the true lumen and one from the false lumen. To cut the septum, an electrification of the wire was performed with cautery (60) and the two catheters fixed to the guidewire were pulled down to the desired level of septum section till the infrarenal segment (Figure 2).
- After septostomy, thanks to IVUS guidance, we double-checked the enlargement of the aortic paravisceral space and the origin of major vessels to assess their patency; subsequently, we performed a comprehensive aortography to evaluate distal visceral and renal vessel perfusion (Figure 3).
- A standard, off-the-shelf inner branch device (E-nside 38-26-22, Artivion) was deployed at the desired position, with full spontaneous expansion and non-upward slip of the graft or malrotation.
- CT, SMA, and true lumen of the LRA were sequentially cannulated and stented with Covera self-expandable covered bridging stents (Figure 4). The RRA and its branch were embolized using a Concerto coil and 2 Ampltzer plugs into the respective inner branch.
- Distally, an EVAR (Gore Excluder CXT 321414E) was implanted in a standard fashion; the right distal iliac sealing was completed with an appropriate sized iliac limb extension; distal exclusion of the left limb was not performed to maintain temporary reperfusion of the sac and favour adequate spinal cord adaptation.
Video 3. Three minute video showing focal points of the entire procedure.
Get the latest clinical cases and breaking news delivered straight to your inbox!



